August 20, 2019
You may have tuned into my recent podcast episode “Peptides Unveiled: The Best Peptide Stacks For Anti-Aging, Growth Hormone, Deep Sleep, Hair Loss, Enhanced Cognition & Much More!“. If you haven't, I'd recommend you take a listen. One of my guests on that podcast episode was Jeremy Delk, who is at the helm of Tailor Made Compounding, one of the safest places to procure peptides under the supervision of a physician—especially compared to the host of websites that sell suspect, often tainted or useless versions of these powerful molecules.
One of Jeremy's colleagues at Tailor Made is Ryan Smith (pictured above), vice president of the company, a guy who is knee-deep in peptide research, and one of my go-to resources when I have questions about peptides.
While of course it would be to Ryan's benefit to only focus on the positives of peptides, I have appreciated his frank honesty with me in the past about the potential risks of some of these compounds. So when he shared with me his concerns about the role of peptides in oncogenesis and cancer, I asked him if he could write a few of his thoughts for my audience, and he generously obliged.
Below are words of wisdom from Ryan about peptides, particularly those that you should be concerned about regarding dosing and frequency if you want to strike the sweet spot between efficacy and risk. Enjoy, and feel free to leave your thoughts, questions, and feedback below for Ryan or I to respond to.
Are Peptides Safe? Their Role in Oncogenesis and Cancer
When people discuss peptides, the conversation almost always starts with the most well known and widely used products in the field of optimization and performance. GH secretagogues like CJC, ipamorelin, tesamorelin, and repair and recovery products like BPC-157 and thymosin beta-4 are almost always lauded with passionate statements of their therapeutic activity.
While these therapies are extremely exciting for their potential to help heal the body and promote optimal health, they are also complicated pleiotropic (producing multiple effects) biological signaling agents that have a multitude of downstream interactions in the body. As a result, these things need to be viewed as more complex molecules with intricate clinical considerations.
One of the largest clinical considerations is how to balance the proliferative or signaling effects of peptides with the possibility of causing the same proliferative effects in tissues which might be cancerous. It has been well documented for instance that higher IGF-1 levels are correlated with shorter life span and increased risk of certain types of cancer like breast, colon and prostate cancer. Knowing the research and risks behind this should be behind every treatment plan.
The Current State and Development of Peptides in Cancer Treatment
Before we talk about the complicated nature of peptides and the problems that they may present in cancer, it is also important to note their exciting potential in the treatment of cancer as well.
As most would probably guess, cancer is the leading cause of death in the industrialized world. While the medical community continues to design innovative drugs and treatment protocols, we are also losing treatment options as current cancer therapies are losing efficacy due to…
- Drug resistance,
- Lack of tumor selectivity,
- Solubility
- Cost
Meanwhile, Peptides provide a promising therapy for cancer due to…
- Ease of synthesis
- High target specificity and selectivity
- Low toxicity and drug to drug interactions
Thus, the amount of oncology peptides approved or in active development is continuously increasing.
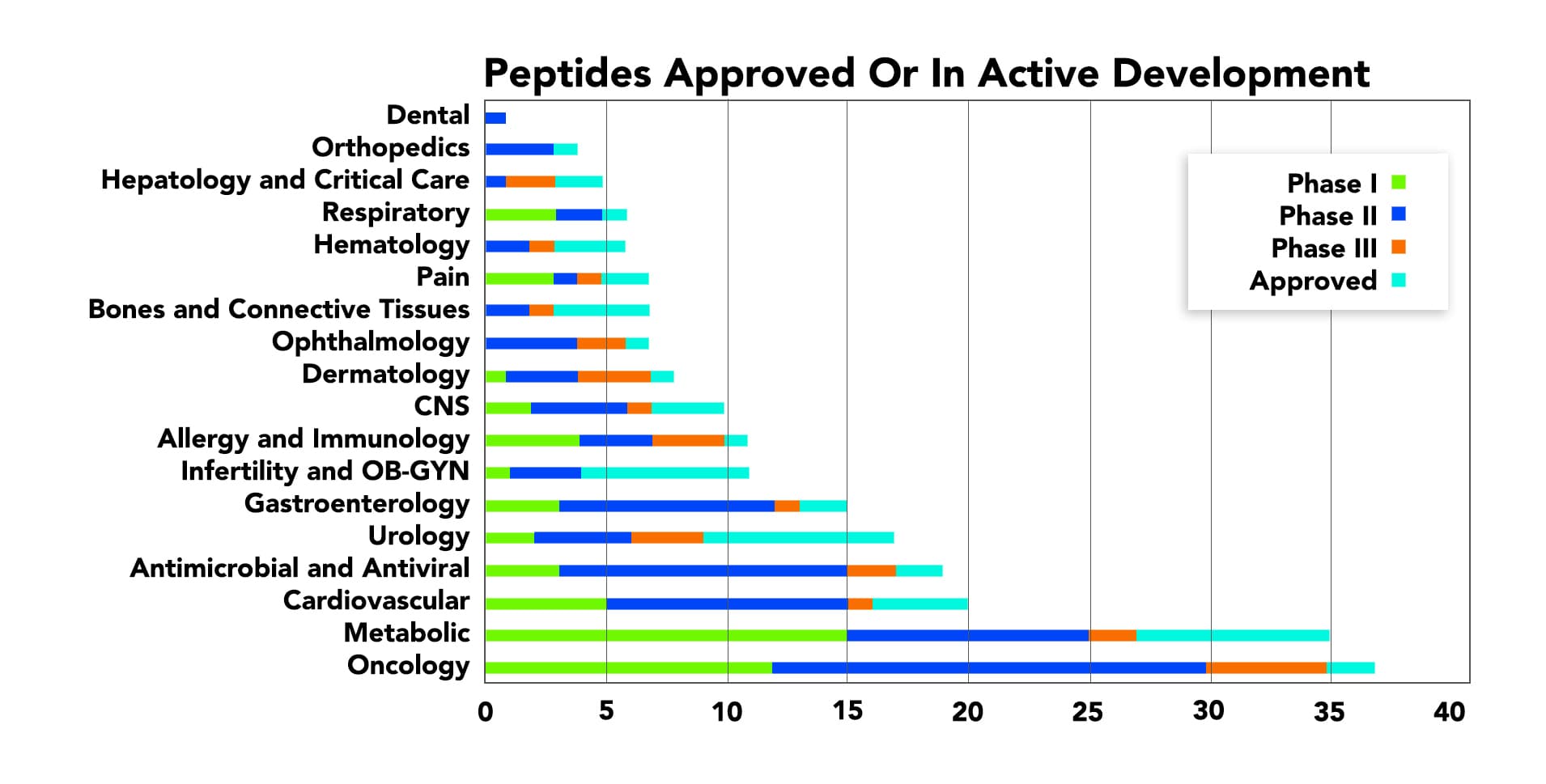
Peptides in Cancer: The Strategies
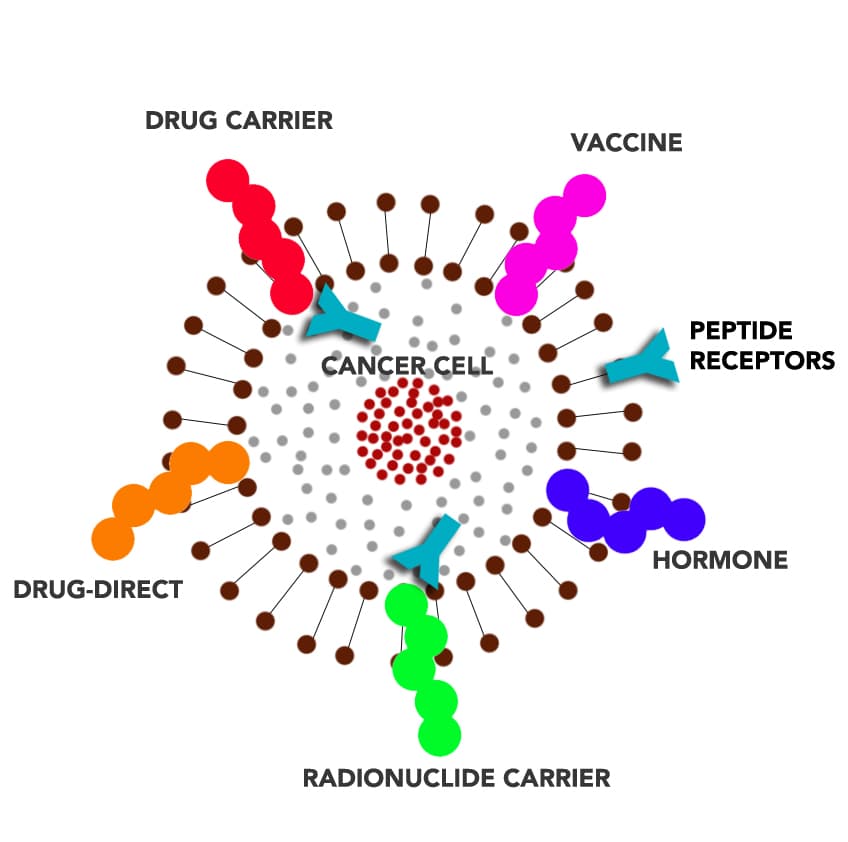
Peptides can be utilized in a number of different ways in treating cancer: peptides directly as drugs (e.g., as angiogenesis inhibitors), tumor-targeting agents that carry cytotoxic drugs and radionuclides (targeted chemotherapy and radiation therapy), hormones, vaccines, or potential diagnostic tools and biomarkers.
Some common peptide drugs that are currently used include bortezomib, mifamurtide, PNC27, SSRB1, iRGD, thymosin Alpha-1, antimicrobial peptides, cell-penetrating peptides, and met-enkephalin.
Although peptides are used as a therapeutic strategy to treat cancer, which peptides should be viewed with caution?
Peptides are extremely selective products with short half-lives and metabolism which most commonly happens in the serum. As a result of these characteristics, their side effects and profile is usually very limited.
The following illustration shows the different ways peptides can be used in cancer treatment; whether as an anti-cancer drug, cytotoxic drug carrier, vaccine, hormones, and radionuclide carrier.
Although peptides are used as a therapeutic strategy to treat cancer, which peptides should be viewed with caution?
Peptides are extremely selective products with short half-lives and metabolism which most commonly happens in the serum. As a result of these characteristics, their side effects and profile is usually very limited (citation and picture). However, contrary to popular opinion, these things are not without side effects and have clinical considerations which often should cause restraint.
In the world of sports performance and health optimization, many of the same peptides which can cause healing, weight loss, and muscle gain have other proliferative effects which should be considered before dosing.
Here we will explore some of the most frequently used peptides by the general public and their downsides as it related to oncogenesis and cancer therapy.
Thymosin Beta 4 (also called TB500)
Thymosin β-4 (Tβ4 or TB-500) has been a product used in sports doping for many years.
However, it was originally discovered in mid-1981, when Dr. Allan Goldstein from the Laboratory of Abraham White at the Albert Einstein College of Medicine in New York isolated it from thymus fraction 5 (TF5).
Several other peptides, including thymosin a1, thymosin β-10, and thymosin β-15, were also isolated from TF5. The biologically active thymosin peptides found in TF5 belong to the family of biological response modifiers (BRMs), which are now known to regulate a large number of immune responses and also participate in the repair and regeneration of tissues following injury.
Structure: Thymosin Beta-4 is a 43 amino acid sequence encoded by the gene TMSBX4 and is present in all human cells. Tβ4 is naturally found in higher concentrations in tissue damaged areas because it is naturally upregulated as a mechanism to repair injury. This peptide has been used frequently in sports doping for the past 20 years for its ability to decrease injury times and reduce delayed onset muscle soreness.
Tβ4 is an extremely pleiotropic product with multiple mechanisms of action due to its multiple active binding sites.
Shown below are biological activities defined by active sites. The sequence of thymosin β4 is shown with the location of active sites indicated. There is also a lot of the activities found in various fragments.
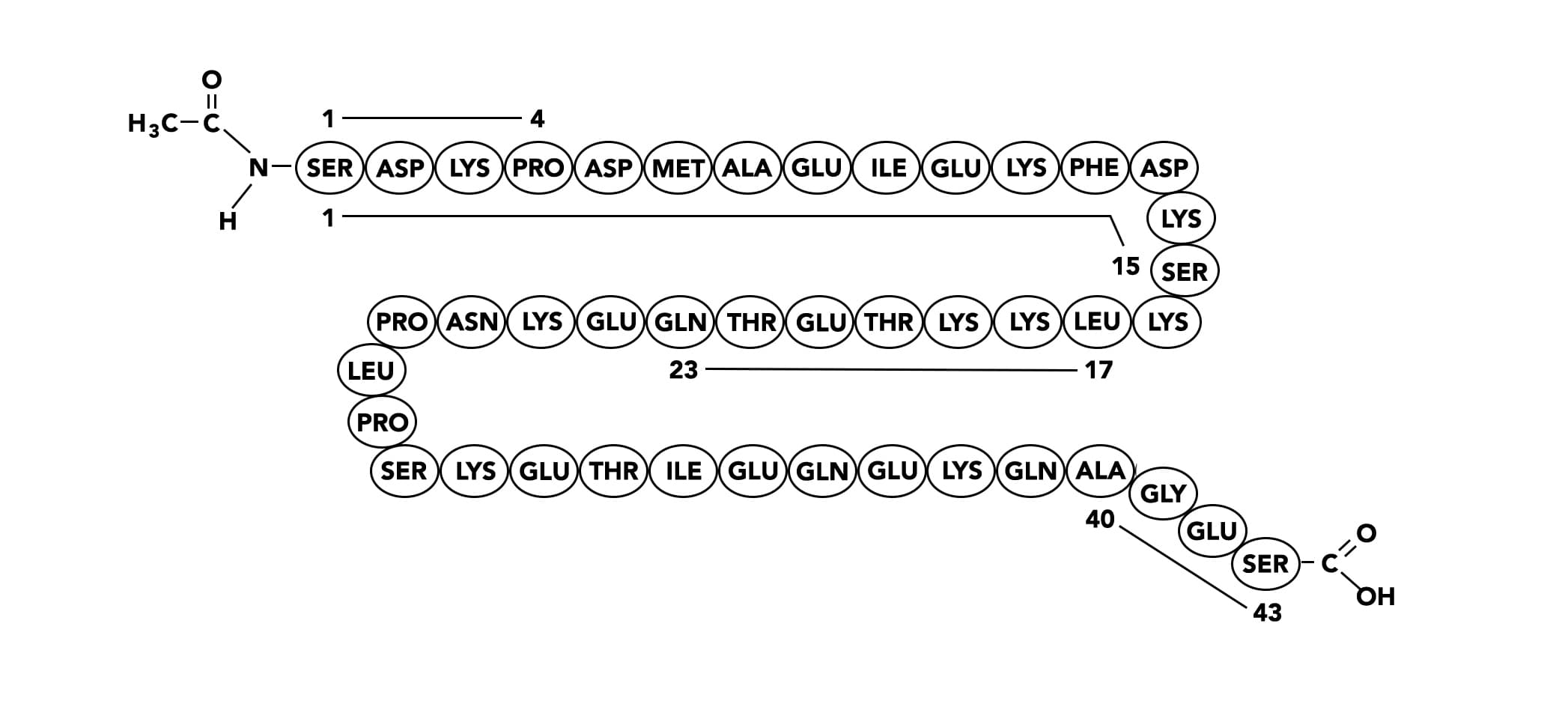 1-4
1-4
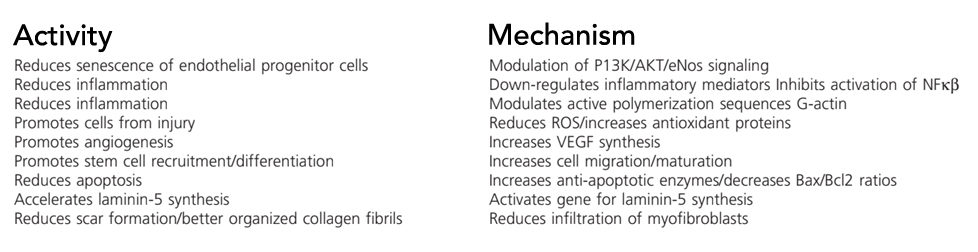
- Anti-inflammatory
- Anti-fibrotic
- Stimulates epicardium-derived progenitor cells
- Inhibits bone marrow cell differentiation
- Decreases TNF-alpha release by macrophages
- Suppresses Smad activation (blocks TGF-β signaling)
1-15
- Anti-apoptotic
- Protects from cytotoxicity
17-23
- Actin binding
- Promotes hair growth
- Improves dermal wound healing
- Stimulates angiogenesis
- Induces mast cell exocytosis
40-43
- Increases embryonic cardiac cell migration
- Increases heart function post-ischemia
Biological activities and defined mechanism(s) of action of thymosin β4.
Uses: TB500 peptides has been used frequently in sports doping for the past 20 years for its ability to:
- Decrease injury times
- Reduce delayed onset muscle soreness
It also affects many different tissues in several ways. As a result, it has shown utility in treating many of the following conditions:
- Athletic recovery: preventing delayed onset muscle soreness (DOMS)
- Post-surgery/injury
- Hair loss
- Cardiac healing
- Traumatic brain injury (TBI)
- Lung Inflammation
- Corneal Healing
- Actin Sequestering Protein
Shown below are various organs and the injuries that have been found to be repaired or affected by thymosin β4.
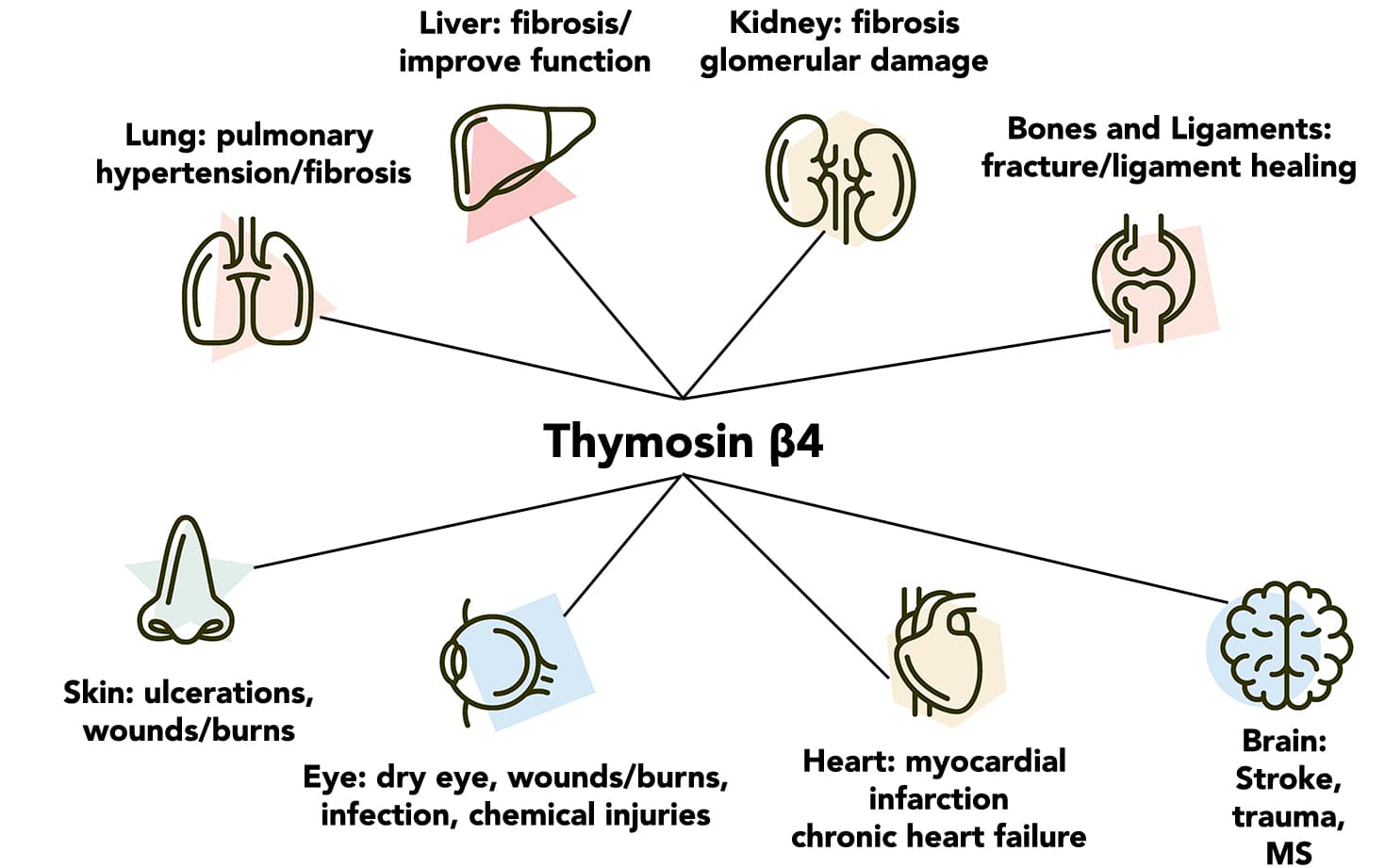
Anecdotal and clinical research has shown its ability to be an amazing medication which can change patients lives in several disease states. It even got Orphan Drug approval in 2013 for neurotrophic keratitis.
Concerns: The research also indicates that despite its positive effects, it might also present concern for its ability to upregulate the ability of cancerous cells to spread. This makes sense due to thymosin β-4’s mechanism of action on cell migration and angiogenesis. Unfortunately, if thymosin β-4 contributes to aberrant cell migration it could contribute to pathologies such as cardiovascular diseases, tumor metastatic cascade including tumor angiogenesis, invasion, and metastasis.
Endogenous upregulation through genetic changes have correlated β-thymosins with cell migration and metastatic potential of human cancers such as melanoma, thyroid carcinoma, colon cancer, and prostate cancer.
| Cancer Types | Tβ4 Findings |
|---|---|
| Colon and Gastric | Clinically, CRC patients with high Tβ4 expression levels are correlated with a marked increase in the incidence of subsequent distant metastasis, shorter disease-free, and low overall survival rates |
| Melanoma | Tβ4 expression is elevated in metastatic melanoma compared with nonmetastatic melanoma counterparts and Tβ4 was associated with induction of VEGF expression which can cause increase the spread of cancer |
| Fibrosarcoma | Fibrosarcoma cells with highly metastatic potential express high levels of Tβ4, whereas fibrosarcoma cells with low metastatic potential express little or no Tβ4 (115). |
| Other Types | Compared with adjacent normal tissues, overexpression of Tβ4 and Tβ10 are seen in human esophagus cancer, pan-creatic cancer, and renal cancer, as well as in human cholangiocarcinoma and neuroblastoma. |
| Roles and mechanisms of β-thymosins in cell migration and cancer metastasis |
With all of this research, it is clear that some cancers might upregulate Tβ4 production as a way to increase its ability to spread and grow. Still, that doesn’t stop it from being a great clinical resource. In the human studies which have been done, the risk has been acknowledged but closely followed.
A phase 1a and 1b review said the following:
“The reported potential of Tβ4 to influence the metastatic potential of certain malignancies through its ability to promote angiogenesis and stimulate cell migration warranted close follow-up for any potential cancers. No cancers were identified during a 6-month follow-up period.”
Thymosin β-4 is absolutely contraindicated for anyone with cancer. At the cellular level, it has been linked to increased risk of metastasis, but there has been no link to exogenous administration and increased cancer risk yet due to lack of study. It may have a proliferative risk when used continuously in cancer therapy but more research needs to be done.
GH Secretagogues
Growth hormone has long been billed at the anti-aging hormone due to its wide-reaching effects on the body in many areas of aesthetic appeal.
However, adult growth hormone deficiency (AGHD) is a medical diagnosis and could cause a series of abnormal manifestations, such as increased body lipid profile, abdominal obesity, impaired glucose tolerance, increased mortality risk of cardiovascular diseases, and many more.
Research: Growth hormone replacement therapy (GHRT) has been carried out extensively in the last 20 years and almost all studies have suggested considerable therapeutic benefits with improvements in lowering the risk of cardiovascular, exercise performance, and quality of life.
Concerns: The link to growth hormone and cancer risk has been widely debated. The hypothesis about the risk of cancer in patients treated with growth hormone was based on the ideas that growth hormone and IGF-1 can affect cells by signaling a transduction cascade for the cells' proliferation and survival. This cell survival is good for most cells but could possibly keep a cancer cell alive even though the body should cause it to die through apoptosis.
While there is strong evidence from animal and cell experiments suggesting that growth hormone and IGF-1 were involved in the occurrence and development of tumors, such evidence in human studies remains unclear. Even further, several observational studies have suggested an association between IGF-1 level in circulation and a risk of some cancers, such as breast cancer and colorectal neoplasms.
As GH secretagogues like CJC 1295, tesamorelin, MK-677, and ipamorelin all encourage higher levels of GH secretion and higher IGF-1, this question still remains. While most recent meta-analyses show no risk, it is still an important clinical consideration.
Dihexa
Dihexa is a peptide variant derived from angiotensin IV, which has been found to potently improve cognitive function in animal models.
Dihexa has been shown to enhance acquisition, consolidation, and recall of learning/memory formation. It was found to be seven orders (10 million) of magnitude more potent than BDNF, brain-derived neurotrophic factor, encoded by the BDNF gene.
Uses: Due to its potency, wide range of application, and its oral bioavailability, it is an exciting drug for the treatment of Alzheimer's, Parkinson's, and many other neurodegenerative diseases.
How it works: Its mechanism of action is thought to mimic a product called hepatocyte growth factor. It does this by activating a receptor called c-Met. c-Met is a receptor tyrosine kinase and is expressed on the surfaces of various cells. The binding of HGF to c-Met initiates a series of intracellular signals that mediate embryogenesis and wound healing in normal cells. However, in cancer cells, aberrant HGF/c-Met axis activation can promote tumor development and progression by stimulating the PI3K/AKT, Ras/MAPK, JAK/STAT, SRC, Wnt/β-catenin, and other signaling pathways.
Concerns: However, there is no clinical or even animal evidence to suggest a link between dihexa at the moment and cancer. In the video below, Dr. Harding, the developer of this peptide, says there is no link. Additionally, their patent says the following “Of particular note is a lack of neoplastic induction since c-Met is recognized as an oncogene. This is unsurprising since oncogenesis requires multiple mutations including both oncogene induction and tumor suppressor attenuation”.
LL-37
Natural antimicrobial peptides (AMPs) like LL-37 are universal host defense molecules that have remained potent through millions of years of evolution. More than 1,770 AMPs from bacteria, fungi, plants, and animals were registered in the Antimicrobial Peptide Database (APD) as of August 2011.
Usually, these peptides can rapidly eliminate invading bacteria by disrupting their cellular membranes, making it difficult for bacteria to develop resistance.
Discovery: In 1903, the Nobel Prize in Physiology or Medicine was awarded to Niels Ryberg Finsen, who found that concentrated rays from carbon arc lights were effective in treating lupus vulgaris—a skin infection by Mycobacterium tuberculosis. In the 1920s and 1930s, the ‘sunshine vitamin’ was finally isolated and a mechanistic relationship between sunlight and vitamin D was described as exposure of skin to ultraviolet (UV) radiation converted 7-dehydrocholesterol to vitamin D. Now, we know that light triggers the synthesis of vitamin D, which induces the expression and release of human LL-37, which kills the bacterium.
Uses: LL-37 is the only cathelicidin AMP found in humans. LL-37, like cathelicidins, is stored in neutrophil granules as inactive precursors and released as mature peptides when neutrophils are stimulated by sensing a foreign pathogen. In addition to its broad spectrum of bactericidal activities, LL-37 has a wide range of inflammatory/immune-modulatory actions. It also fights viruses and fungi as well and is found mainly expressed in the intestines.
Besides fighting off pathogens, LL-37 has also been hypothesized to have a functional role in Alzheimer’s prevention, reducing scleroderma, helping with SIBO and other types of GI function, and many more diverse indications. However, its clinical use has been limited because it can have opposite and contradicting effects in certain tissues.
Concerns: There is data that it can help some autoimmune issues like scleroderma, but make other autoimmune issues like psoriasis worse. Cancer is another example of this contradictory effect. LL-37 induces tumorigenic effects in ovary, lung, breast, prostate, and pancreas cancer, as well as in malignant melanoma and skin squamous cell carcinoma. In contrast, LL-37 exhibits an anti-cancer effect on colon cancer, gastric cancer, hematological malignancy, and oral squamous cell carcinoma.
Many have postulated that the reason for these contradictions is because of tissue-dependent receptor changes. LL-37-targeted receptors include at least four G protein-coupled receptors (GPCRs), three receptor tyrosine kinases (RTKs), a ligand-gated ion channel (LGIC) and toll-like receptors (TLRs).
Overall, LL-37 is an extremely interesting peptide, but it can increase the risk of some cancers and decrease the risk of others. Even though it is a natural product, it has no human clinical trials outside of topical application and should also be viewed with caution.
Summary
Peptides have proven their worth in the growing science of integrative medicine. They are favorable therapeutic drug candidates that have drastically improved the healthcare industry. However, their effect on the body is highly diverse and tissue-specific. As a result, all peptides need to be looked at closely and with medical expertise before administration.
In other words, when it comes to peptides, always work in close conjunction with a physician to…
…dose correctly…
…assess your personal risk…
…and don't assume the same peptide protocol presents the same risks and benefits for everyone…
For more, listen to Ben's recent peptide podcast with Dr. William Seeds and Jeremy Delk here, and leave your questions, comments, and feedback below.














I have Pulmonary Arterial Hypertension and a provider prescribed injectable copper peptides for me. Do you agree that this could possibly help? Also, are there any risk? Also, I no longer have access to her services and plan to go with another functional medicine provider. We are having a difficult time finding a pharmacy that makes this. Any suggestions? I live in Alabama.
would a synthetic peptide behave the same way as a native peptide in the body assuming they have the identical amino acid composition?
After I originally commented I seem to have clicked on the -Notify me when new comments are added- checkbox and from now on every
time a comment is added I get 4 emails with the exact same comment.
Perhaps there is an easy method you can remove me
from that service? Cheers!
how to to take Dihexa ? can it be injected if yes then any specific area?
I see a company tb-500.com which is called thymosinlabs selling an oral version of Tb-500 and bpc157 and I am wondering if these compounds inside of a capsule are a legit way to go ?
Are you able to share the data about LL-37 helping with Scleroderma? Was this a research study or something said by Ryan?
Yes, please point to the evidence re: BPC-157 exacerbating pre-existing cancers. This is contrary to what I have read. Thank you!
Could I get the cliff notes? Specifically advice regarding Ipamorelin and semorelin?
Did you get these? I’m also interested.
does this apply to collagen peptide powder?
Should one use caution with items like
Vital proteins collagen peptides? I was recently diagnosed with breast cancer at the age of 39 and my genetics were negative for the brca gene.
Since BPC 157 is so popular, I would also mention its risks with existing cancers as well. Due to increased angiogenesis, tumor or cell growth can also be increased.
BPC-157 so far only has anti-cancer evidence
You have misunderstood what I wrote. Yes, it can help prevent some cancers. It can also exacerbate a pre existing cancer thru angiogenesis.
Can you point me to the evidence? BPC-157 is of particular interest to me. Thanks!
No, the BPC-157 anti-cancer evidence is for *existing* cancers.
I don’t think cancer prevention has been shown.
This in-vitro against melanoma is easy to find and show, mouse ones are listed in patent in a very confusing way, I don’t have them at hand.
BPC 157 inhibits cell growth and VEGF signalling via the MAPK kinase pathway in the human melanoma cell line. Radeljak et al, Melanoma Research: August 2004 – Volume 14 – Issue 4 – pp A14-A15
http://doi.org/10.1097/00008390-200408000-00050