October 13, 2020
As you probably already know, some of the major suppliers of peptides and innovative molecules in the medical space have recently had to pump their brakes on production after receiving legal notices regarding the compounding of products that have not been approved by the FDA, and this includes peptide manufacturers I've discussed on podcasts and in articles before, such as Tailor Made Compounding.
In other words, popular peptides that a ton of folks in the functional medicine, health, and fitness space use—such as CJC 1295, Ipamorelin, Selank, Semax, and many others—are no longer able to be legally prescribed by doctors. (You can check out the bulk drug substance list on what is now able to be compounded here.)
But wait, what's a peptide?
If you're unfamiliar with these magical little molecules, you may want to access the following podcasts and articles to bring yourself up to speed:
- Demystifying Growth Hormone-Releasing Peptides – Everything You Need To Know About GH, Ipamorelin, Tesamorelin & More!
- Peptides Unveiled: The Best Peptide Stacks For Anti-Aging, Growth Hormone, Deep Sleep, Hair Loss, Enhanced Cognition & Much More!
- The Dark Side Of Peptides: Why You Need To Proceed With Caution When Using These Powerful But Potentially Carcinogenic Molecules.
- The Peptides Podcast: Everything You Need To Know About Anti-Aging, Muscle Gain, Fat Loss & Recovery Peptides.
- The Little-Known Russian Wonder Compound & The Fringe Future Of Anti-Aging Medicine.
- How To Use BPC-157: A Complete Dummies Guide To Healing The Body Like Wolverine.
- How To Use Growth Hormone Stacks For A Better Body: Everything You Need To Know About IGF-LR3, GHRP, and GHRH Peptide Stacks.
Anyways, back to this recent FDA decision about peptides. To make matters worse, on March 23rd of this year, the FDA also supported a legal decision that changed the definition of what is considered a biologic drug. Because of this, many FDA-approved products that compounding pharmacies have been making for years, such as HCG (another very popular compound in the hormone replacement industry) are now totally unable to be legally compounded.
The FDA had previously stated its interpretation of the statutory terms “protein” and “chemically synthesized polypeptide” in the amended statutory definition of “biological product.” The FDA interprets the term “protein” to mean any alpha amino acid polymer with a specific defined sequence that is greater than 40 amino acids in size. Alternatively, the FDA previously interpreted the term “chemically synthesized polypeptide” to mean any alpha amino acid polymer that is made entirely by chemical synthesis and is greater than 40 amino acids, but less than 100 amino acids in size.
A “chemically synthesized polypeptide” was not a “biological product” and was not going to be regulated as a drug under the FD&C Act unless the polypeptide otherwise met the statutory definition of a “biological product.” This definition was scientifically appropriate, but at the last minute, the latest appropriations bill (enacted in December of 2019) further amended the definition of “biological product” to remove “(except any chemically synthesized polypeptide).” Now, in the definition documented below, there is no differentiation between protein and polypeptide.
Biological Product – A virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, protein (except any chemically synthesized polypeptide), or analogous product, or arsphenamine or derivative of arsphenamine (or any other trivalent organic arsenic compound), applicable to the prevention, treatment, or cure of a disease or condition of human beings.
The term “biosimilar” or “biosimilarity” in reference to a biological product means…
-
- That the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components; and
- There are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product.
As a result of these changes, drugs that are synthetically created are being regulated as much more complicated biologics.
This is unfortunate as it takes away the ability for compounding pharmacies to make much-needed variations on products. For instance, an AIDS patient with an allergy to mannitol is now unable to receive standard of care medicine due to their allergy. The same goes for patients who might have a benzyl alcohol allergy. Benzyl alcohol is commonly used as a preservative, and now anyone who has an allergy to it can no longer legally get safe and efficacious medicine prepared by a compounder.
The original law was intended to make it easier for similar drugs to get FDA approval, but by removing the statement about chemically synthesized polypeptides at the 11th hour, it disenfranchised thousands of people from getting the medication they need. This includes products such as Tesamorelin and HCG, which are now 5-6x more expensive (if you can even purchase them at all).
With all that has happened, many people believe peptide medicine is no longer a viable option for themselves or their patients. However, there are still amazing peptide developments in the pipeline I believe the world should know about. So, despite the pesky recent activity of the FDA, in today's article, you'll discover nine such peptides that you can still get your hands on from a physician (I recommend the International Peptide Society), how they work in your body, what research has shown them to be beneficial for, and much more.
#1: ARA 290 – Pain, Peripheral Neuropathy, Cholesterol, Blood Sugar, Repair, & Recovery.
- Sequence/Structure: {Glp}-Glu-Gln-Leu-Glu-Arg-Ala-Leu-Asn-Ser-Ser
- Molecular Weight: 1257.31 g/mol
- Molecular Formula: C₅₁H₈₄N₁₆O₂₁
- Dosing: 4mg subcutaneously daily for 30 days.
Erythropoietin (EPO) is widely known as a doping agent for endurance sports. Despite its negative connotation, EPO is an indispensable medical product that can help in treating a wide range of ailments including anemia resulting from chronic kidney disease; chemotherapy-induced anemia in patients with cancer; a host of GI-related diseases such as inflammatory bowel disease, ulcerative colitis, and Crohn’s; and even can help with myelodysplasia from the treatment of cancer.
Over the past 15 years, preclinical studies have demonstrated another interesting use for EPO that isn’t often talked about. Over this time, scientists have identified the presence of an endogenous protective system that is activated by inflammation, metabolic stress, and tissue injury. The receptor for this response, innate repair receptor (IRR) is a member of the type I cytokine receptor family and is a complex consisting of βcR (CD131) and EPO receptor subunits.
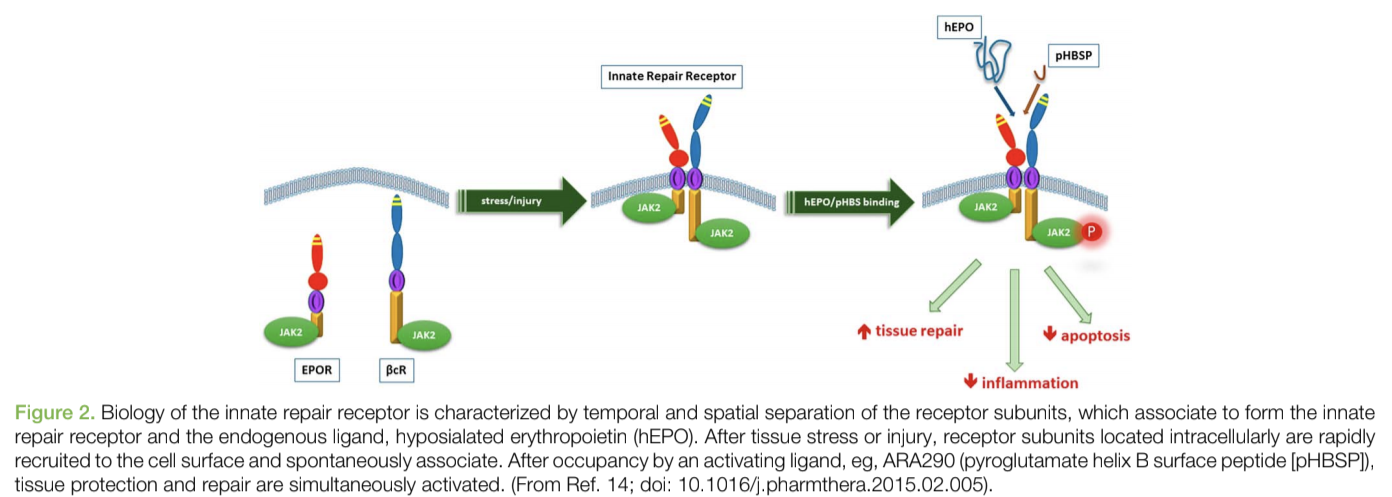
This homeostatic repair response is upregulated and activated by hypoglycosylated EPO produced in situ by many cells as a stress response. Locally produced EPO has been shown in diverse model systems to antagonize the production and effects of proinflammatory molecules (for example, tumor necrosis factor) and activate healing processes.
If some individuals don’t produce enough of this local EPO, they might not be able to compensate for high levels of inflammation and tissue damage. As a result, supplementing with something like EPO might help with repair and recovery in addition to its effects on the hemopoietic system.
However, many patients who have a need for the localized response can also be hurt by the systemic effects of EPO. Therefore, the use of recombinant human erythropoietin (rhEPO) for the treatment of tissue injury is limited by the potential side effects of increased erythrocyte mass and endothelial cell activation, which, in combination with activated platelet production, predisposes to thrombosis (blood clotting).
As a result, scientists wanted to create a variant of EPO that could have local effects on the IRR without the systemic effects.
They were successful in creating a remarkable peptide that could do this called ARA 290, or Cibinetide. ARA 290 is an 11-amino acid peptide modeled from the three-dimensional structure of helix B of the EPO molecule that interacts with the IRR. ARA 290 has been evaluated extensively in a wide spectrum of preclinical models and has been shown to help with:
- Diet-induced insulin resistance
- Diabetic retinopathy
- Diabetic autonomic neuropathy
- Myocardial infarction
- Chronic heart failure
- Burns
- Traumatic brain injury
- Shock-induced multi-organ failure
- And more
The results of these studies show that ARA 290 prevents tissue injury, reduces inflammation, and activates healing. However, in contrast to rhEPO that has shown side effects in clinical trials, ARA 290 has been proven safe when formally evaluated in preclinical animal toxicology, normal human volunteers, and patients with sarcoidosis.
In addition to the overwhelming data showing that this peptide is a great addition for patients with neuropathic pain, it has also shown to reduce HbA1C (blood sugar) levels. In one study, after a month of dosing, HbA1c was decreased by around .3 points at day 28 and this was maintained at least 4 weeks following dosing.
ARA290 has also exhibited positive effects on cholesterol. Administration at 4mg daily exhibited a favorable increase in cholesterol/HDL ratio, HDL, and a decrease in triglycerides. It also had amazing results on nerve fiber regeneration and reductions in pain.
The implications of this medication are potentially very broad—it is a great peptide for treating pain, but it can also have cardiometabolic effects. It is also effective at reducing inflammation in general and combatting the inflammation process as well.
#2: Tesofensine – Weight Loss & Appetite Suppression
- Sequence/Structure: (1R,2R,3S,5S)-3-(3,4-dichlorophenyl)-2-(ethoxymethyl)-8-methyl-8-azabicyclo[3.2.1]octane
- Molecular Weight: 520.4 g/mol
- Molecular Formula: C23H31Cl2NO8
- Dosing: 500mcg-1mg orally once daily in the morning.
Although not a peptide, Tesofensine has proven to be extremely effective for weight loss, is orally bioavailable, and very stable. A small molecule that is a noradrenaline, serotonin, and dopamine reuptake inhibitor, it was originally studied for Alzheimer's. However, Tesofensine's application to neurodegeneration was ended when the test subjects started losing too much weight, so it is now used for weight loss. One study showed an average weight loss of 26 pounds over 6 months, and it was able to produce 10% greater weight loss than placebo after 24 weeks of 0.5 or 1.0 mg daily. This is about twice that of currently approved drugs!
Appetite was also significantly suppressed after an overnight fast after treatment with Tesofensine in this study. Primarily acting as an appetite suppressant, Tesofensie also acts by increasing resting energy expenditure as is documented with some of the data shown in the graph below.
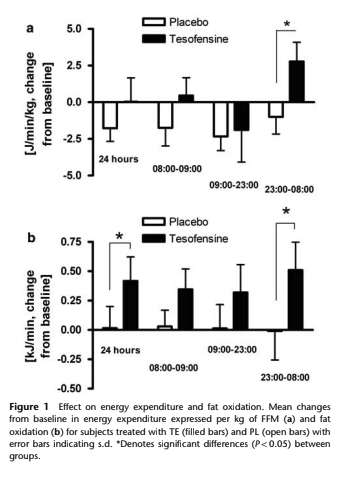
Tesofensine has a very long half-life of about 8 days in humans and the clearance after oral administration is low (30–40 ml/min-1). In most clinical trials, it is usually dosed for 3-6 months. Phase 2 trials for the treatment of obesity have been successfully completed and look very promising.
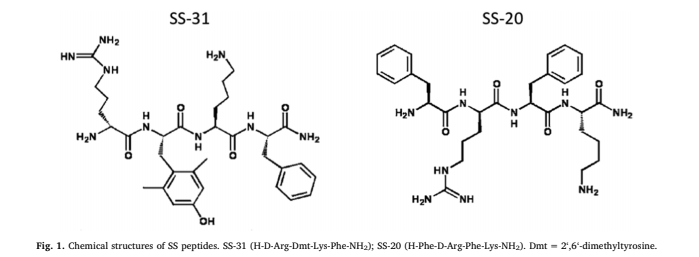
#3: SS-31 – Aging, Sarcopenia, & Performance
- Sequence/Structure: (D-Arg-Dmt-Lys-Phe-NH2; Dmt=2’,6’-dimethylTyr)
- Molecular Weight: 639.8 g/mol
- Molecular Formula: C32H49N9O5
- Dosing: 40mg subcutaneously daily.
Mitochondrial dysfunction is one of the nine hallmarks of aging. Despite the many approaches being pursued for mitochondrial function, none of them directly target the electron transport chain (ETC) to improve coupling efficiency and increase ATP production. Electron transfer and ATP production require numerous proteins to work in harmony on the inner mitochondrial membrane (IMM).
This is an especially important consideration in skeletal muscle as mitochondria play a central role in skeletal muscle health. Sarcopenia is a complex disease involving diverse age-related changes in metabolism and proteostasis, signaling pathways, chronic inflammation, disruption of neuromuscular junctions, and excitation-contraction defects.
Mitochondrial dysfunction is involved in all of these processes as either a cause or consequence of these age-related changes.
This central role of mitochondria in skeletal muscle health is due to their function as both the primary source of adenosine triphosphate (ATP) to meet the sustained energetic demand for muscle contraction and cell maintenance and their role as one of the main sources for cellular reactive oxygen species production. Reduced quality of mitochondria in aging skeletal muscle is manifested by a decline in ATP production accompanied by elevated oxidant production that disrupts both energy and redox homeostasis.
This age-related decline is often related to the IMM. While the typical IMM is very curvy, with the protein machinery of the electron transport train in the curved portions, this curvy appearance flattens out with age. This makes the constituents of the ETC further apart and less efficient at making ATP.
One of those proteins is cardiolipin, which is a phospholipid normally only found in mitochondria and is highly enriched in the IMM, the site of its de novo synthesis. The specific fatty acids on cardiolipin are tissue-specific. In high-energy tissues like the heart, the majority of cardiolipin is composed of linoleic acid. In the brain, the fatty acid composition is more diverse.
Cardiolipin has a unique conical shape that aggregates into non-bilayer structures and causes the IMM to buckle and form cristae curvatures. Deficiency in cardiolipin results in absent or disorganized cristae structures and impairs ATP production.
In addition to cristae formation, cardiolipin is required for the stability and activity of the protein complexes on the IMM. Cardiolipin integrates within all respiratory complexes and is required for optimal activity of complex I, III, IV, and ATP synthase. In turn, membrane proteins help to shape the morphology of the IMM.
Essential for the formation of characteristic cristae structures is the mitochondria contact site and cristae organizing system (MICOS) complex residing at the cristae junctions. The ATP synthase is localized to cristae tips where cardiolipin is concentrated, and the dimerization of ATP synthase helps stabilize cristae curvatures and optimize its own activity.
In other words, cardiolipin is the “glue” that stabilizes your IMM for optimal energy production, but becomes oxidized and less effective as you age.
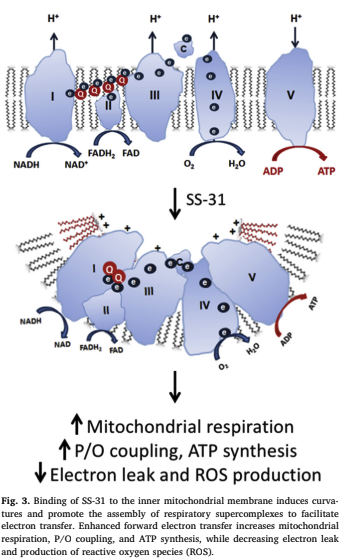
The oxidation of cardiolipin disturbs cardiolipin microdomains on the IMM and results in loss of cristae curvatures. Loss of cardiolipin destabilizes respiratory supercomplexes and further increases electron leakage and ROS production. While the deterioration of the IMM seems to be inevitable with age, now there appears to be something that can help fix it: SS-31.
SS-31 belongs to the Szeto-Schiller (SS) family of peptides. Hazel H. Szeto, MD, Ph.D., was researching the ability of one of these peptides to bind to the mu-opioid receptor and accidentally discovered its potential for crossing the blood-brain barrier. Subsequent studies showed that SS-02 could readily cross cell membranes and target the IMM. Eventually, the peptide analog SS-31 was designed to selectively target the IMM, while having a negligible effect on opioid receptors.
The SS family of peptides are synthetic tetrapeptides with an alternating aromatic-cationic motif. There are a number of surprising features about these peptides. Despite being highly water soluble with a 3+ net charge, they are readily taken up by all cells via passive diffusion. The mechanism behind their cell permeability is unclear, but the aromatic rings may serve as electron cages to shield the cationic charges via cation-π interaction. These SS peptides may be viewed as “cloaked” or “stealth” as they can evade cellular membranes and even penetrate cell barriers with tight junctions, including the blood-brain barrier; hence the name of the pharmaceutical company that now owns this molecule, Stealth Biopharma.
SS-31 binds cardiolipin on the IMM and is able to promote electron flux in the ETC. The addition of SS peptides to isolated mitochondria increases oxygen consumption and ATP production regardless of whether CI or CII substrates are used. Electrotransfer from CIII to CIV via cytochrome c is considered the rate-limiting step in the ETC. Electrostatic interaction with cardiolipin is advantageous for keeping cytochrome c close to the respiratory complexes. The implications of this are diverse and exciting as SS-31 has been shown in some studies to help in the following areas:
- Traumatic brain injury
- Cognitive deficiency
- Diabetes-related visual decline
- Pulmonary hypertension
- ALS
- Atherosclerosis and cardiovascular disease
- Glaucoma
- Exercise intolerance
There are obvious implications for aging, but there are also very exciting possibilities related to physical performance. Perhaps even more surprising is that correcting some of these age-related deficits could be accomplished with just a single injection…
 A single treatment of SS-31 (3 mg/kg) in aged mice (27 months) rapidly reversed the decline in mitochondrial function. SS-31 is rapidly taken up by skeletal muscle, with maximal levels observed 30 minutes after subcutaneous administration. Maximal ATP production and oxidative phosphorylation coupling returned to levels seen in 5-month-old mice within one hour after SS-31 treatment. SS-31 also restored maximal ATP production in aged mice but had no observable effect in young mice. The improvement in bioenergetics was associated with improved fatigue resistance. Treatment with SS-31 for eight days significantly improved endurance capacity in the aged mice.
A single treatment of SS-31 (3 mg/kg) in aged mice (27 months) rapidly reversed the decline in mitochondrial function. SS-31 is rapidly taken up by skeletal muscle, with maximal levels observed 30 minutes after subcutaneous administration. Maximal ATP production and oxidative phosphorylation coupling returned to levels seen in 5-month-old mice within one hour after SS-31 treatment. SS-31 also restored maximal ATP production in aged mice but had no observable effect in young mice. The improvement in bioenergetics was associated with improved fatigue resistance. Treatment with SS-31 for eight days significantly improved endurance capacity in the aged mice.
A recent clinical study confirmed that SS-31 significantly increased skeletal muscle maximal ATP production in healthy elderly adults. Remarkably, the increase in ATP production achieved with one dose is comparable to that achieved with 6 months of endurance training.
These studies demonstrate the feasibility of rapidly improving exercise performance in the elderly by increasing mitochondrial coupling to maximize ATP production.
#4: Larazotide – Celiac Disease & Gut Dysfunction
- Sequence/Structure: H-Gly-Gly-Val-Leu-Val-Gln-Pro-Gly-OH
- Molecular Weight: 725.845 g/mol
- Molecular Formula: C32H55N9O10
- Dosing: 1-4mg, three times daily
For decades, the pathogenesis of a variety of human diseases has been attributed to increased intestinal paracellular permeability even though scientific evidence supporting this hypothesis has been unsubstantiated. Nevertheless, during the past decade, there have been a growing number of publications focused on human genetics, the gut microbiome, and proteomics suggesting that loss of mucosal barrier function (particularly in the gastrointestinal tract) may substantially affect antigen trafficking—ultimately causing chronic inflammation, including autoimmunity in genetically predisposed individuals.
The gut mucosa works as a semipermeable barrier in that it permits nutrient absorption and also regulates immune surveillance while retaining potentially harmful microbes and environmental antigens within the intestinal lumen. However, this can be disrupted in many diseases and nutritional states.
Celiac disease (CD) is a systemic, immune-mediated disorder triggered by gluten and associated with altered gut permeability. Many studies have shown that gliadin, a component of gluten, is implicated in CD pathogenesis. They believe it is capable of disassembling intercellular junctional proteins. When this happens, the tight junctions that control what is allowed in and out of the epithelial layer become dysfunctional, leading to inflammation and a leaky gut.
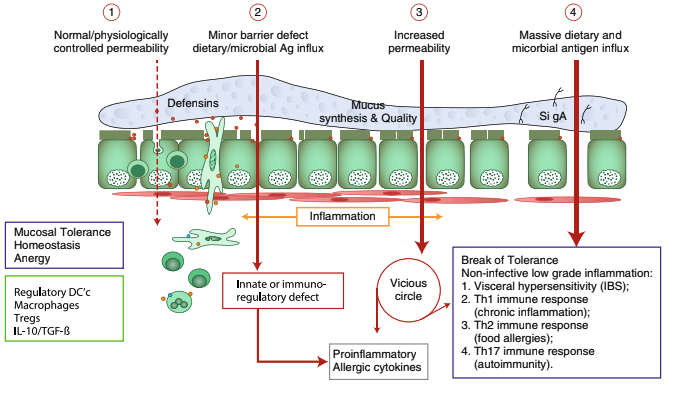
Larazotide is an orally bioavailable peptide that ensures tight junctions are regulated appropriately in order to prevent a permeable and leaky gut. The company bringing this drug to market has completed many successful studies. They have reported positive results from a double-blind, placebo-controlled, Phase 2b trial in February 2014:
“This evaluated the efficacy and safety of larazotide acetate in 342 celiac disease patients who had symptoms despite being on a GFD. The trial consisted of a four-week placebo run-in, 12 weeks of randomized therapy, and four weeks of post-treatment follow-up. Patients were randomized to four groups: a placebo group or larazotide 0.5, 1.0, or 2.0 mg, three times per day. Treatment with the lowest of three doses of larazotide was associated with significant improvement in the primary outcome, i.e. the average on treatment score in the Celiac Disease Gastrointestinal Symptom Rating Scale (CeD GSRS) domains of Diarrhea, Indigestion, and Abdominal pain.”
If you have Celiac disease, gluten intolerance, or leaky gut, this could be an amazing addition to your medication regimen to help reduce symptoms and reverse gut dysfunction.
#5: PTD-DBM – Hair Loss
- Sequence/Structure: Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Gly-Gly-Gly-Gly-Arg-Lys-Thr-Gly-His-Gln-Ile-Cys-Lys-Phe-Arg-Lys-Cys
- Molecular Weight: 3082.66 g/mol
- Molecular Formula: C124H225N61O28S2
- Dosing: Usually this is made at a .5% concentration and applied topically once daily with 5% valproic acid.
PTD-DBM is a peptide that was specifically formulated to target factors that are upregulated in balding individuals. PTD-DBM is a topical hair product that helps activate the Wnt-β-catenin pathway via inhibition of CXXC-type zinc finger protein 5 (CXXC5). This particular pathway has been proven to help rescue DHT induced hair follicle miniaturization. CXXC5 is a negative regulator of the Wnt-β-catenin pathway that has been associated with hair restoration and wound healing.
Often, people will also use micro-needling in order to induce this same pathway. However, studies have shown that PTD-DBM is significantly more effective at inducing hair neogenesis when combined with valproic acid, which stimulates the Wnt-β-catenin pathway when applied topically.
In contrast to many other hair loss products, it has no rebound hair loss if you stop the product and can actually have an effect in regrowing hair quickly (as little as 4 weeks). GHK-cu (which I go over in this article) and PTD-DBM taken together have been shown to be an effective combination for hair loss.
#6: 5-amino-1MQ – Muscle Aging, Repair & Recovery, Physical Performance, Weight Loss
- Sequence/Structure:
- Molecular Formula: C10H11N2
- Molecular Weight: 159.21 g/mol
- Dosing: 100-150mg once daily orally (No published human trials to date.)
Everyone in the preventive medicine space has been inundated with information regarding NAD+, the NAD+ salvage pathway, and NAD+ precursors such as NMN or nicotinamide riboside. You can listen to my podcast “The Next Big Anti-Aging Drug: Everything You Need To Know About “NAD”.” for more information on NAD. However, little is attention is given to an important enzyme that relates to all of this: NNMT.
NNMT is an enzyme that converts nicotinamide to 1-MethylNicotinamide and takes NA out of the NAD+ salvage pathway.
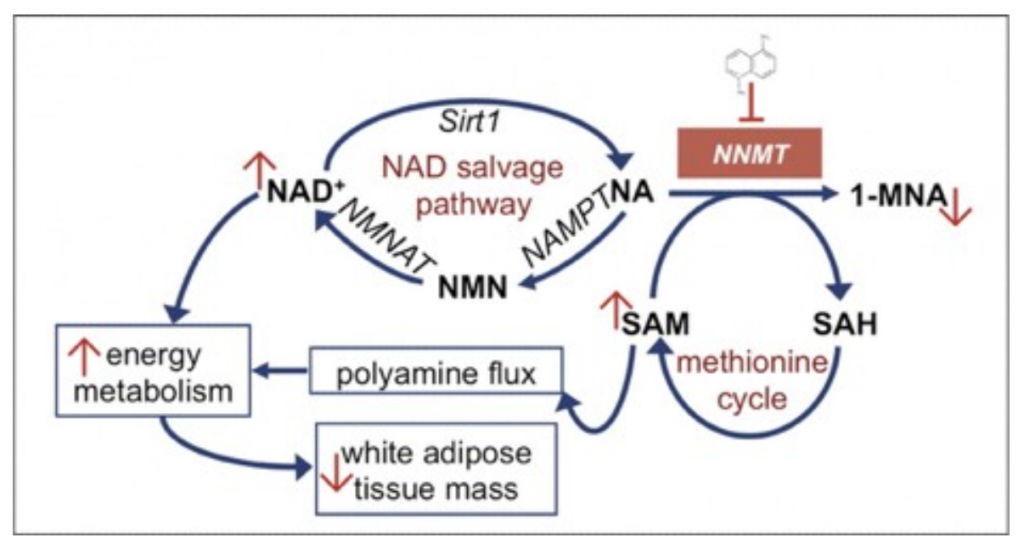
NNMT is predominantly expressed in the liver, but significant levels of the enzyme are also present in other tissues, including adipose, kidney, brain, lung, heart, and muscle tissues.
Enhanced expression and enzymatic activity of NNMT has been linked to a number of chronic disease conditions, making it a significant and relevant target for drug development. For example, several studies have demonstrated a causal relationship between increased NNMT expression and enhanced cancer cell proliferation/progression with potential implications for NNMT as a biomarker for cancer prognosis and a target for anticancer therapeutic development.
NNMT expression has also been reported to be upregulated in patients with Parkinson’s disease, which is suggested to be linked to the production of neurotoxins such as N-methylpyridinium ions that underlie neurodegeneration. Furthermore, and maybe most important, studies in both animals and humans have shown that NNMT expression and activity were increased in obese individuals and those with related chronic metabolic conditions. For instance, we know that NNMT increases with age in fat cells and in muscle cells. In combination with NAD+ precursors like NMN and NR, it has additive effects at promoting sirtuin activation and reducing muscle stem cell senescence. However, several studies have identified a small molecule that can block this enzyme and influence the NAD+ salvage pathway for positive health results: 5-amino-1MQ.
When 5-amino-1MQ was given in animal studies, there was a 30% decrease in adipocyte size and a 40% decrease in adipocyte volume in just 11 days. Plasma lipid-profile measurements showed that the total cholesterol levels were 30% lower in treated DIO mice relative to control DIO mice and it reduced lipogenesis by 50% and 70%, respectively, compared to control.
In muscles, 5-amino-1MQ increases stem cell proliferation to encourage quicker muscle repair and recovery. This improved muscle activity translated not only to larger myofibers after injury but also to greater contractile function, with the peak torque of the TA increased by ~70% in treated mice compared to controls. Anecdotally, it has also been documented to have immediate performance effects in individuals who aren’t injured.
#7: MCC950 – General Inflammation & Inflammatory Diseases
- Sequence/Structure: N-[[(1,2,3,5,6,7-Hexahydro-s-indacen-4-yl)amino]carbonyl]-4-(1-hydroxy-1-methylethyl)-2-furansulfonamide sodium salt
- Molecular Weight : 426.46 g/mol
- Molecular Formula: C20H23N2NaO5S
- Dosing: No Human clinical trials to date.
The discovery of the inflammasome protein complex in 2002 was a breakthrough in the understanding of how the immune system triggers inflammation. Now, researchers are attempting to modulate its activity to treat diseases ranging from inflammatory conditions to diabetes.
MCC950, a small-molecule NLRP3 inflammasome inhibitor, has been shown to improve diabetic vascular endothelial dysfunction. MCC950 is a diarylsulfonylurea-containing compound that was originally shown to block ASC oligomerization and inhibit NLRP3 inflammasome activation. MCC950 attenuated intracerebral hemorrhage against thrombin-induced NLRP3 inflammasome activation and cell apoptosis in microglia.
Moreover, MCC950 treatment decreased neurological impairment, infarction volume, and neuronal apoptosis in a mouse model of ischemic stroke. MCC950 was also shown to improve cognitive function in this model and many others.
#8: TCAP-1 – Muscle Function & Repair
- Sequence/Structure: pGlu-Gln-Leu-Leu-Ser-Thr-Gly-Arg-Val-Gln-Gly-Tyr-Asp-Gly-Tyr-Phe-Val-Leu-Ser-Val-Glu-Gln-Tyr-Leu-Glu-Leu-Ser-Asp-Ser-Ala-Asn-Asn-Ile-His-Phe-Met-Arg-Gln-Ser-Glu-Ile-NH2
- Molecular Weight: 14129.66 g/mol
- Molecular Formula: C584H940N176O185S1Se9
- Dosing: No Human clinical trials to date.
Although TCAP and teneurins have been largely studied in the brain, their role in skeletal muscle has only recently been studied. Skeletal muscle is one of the most important factors in determining the overall metabolism of an organism, which led to the question of whether TCAP-1 has effects on muscle metabolism. TCAP-1 is highly involved in skeletal muscle metabolism and plays a major role in glucose uptake, increased aerobic metabolism, and increased or enhanced muscle function.
Skeletal muscle is a major target of glucose uptake; the role of TCAP-1 can enhance this uptake and increase energy metabolism. In mice, TCAP-1 treatment significantly increased muscle contractile force, prolonged contraction velocity, and relaxation rate during fatigue, indicating an enhanced muscle function. Histological analyses of these muscles indicate that TCAP-1 treatment increases oxidative capacity as observed by significant increases in NADH levels.
It has even been shown that TCAP-1 modulates calcium cycling, where it mediates calcium influx into the mitochondria resulting in mitochondrial depolarization. These actions have been established to increase mitochondrial activation, thereby increasing energy production in the cells.
#9: ALRN-5281 – Growth Hormone
- Sequence/Structure: Tyr-{D-Ala}-Asp-{S5}1-Ile-Phe-Thr-{S5}2-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-{S5}3-Leu-Leu-Gln-{S5}4-Ile-{2-AMINOHEXANOIC ACID}-Ser-Arg-NH2 {S5}1-{S5}2 stapled bond;{S5}3-{S5}4 stapled bond
- MW: 3412.08
- MF: C161H264N42O39
- Dosing: .05-.15mg/kg in human phase 1 trials.
ALRN-1 is the newest of an assortment of growth hormone secretagogue peptides. However, as a stapled peptide (a short peptide, typically in an alpha-helical conformation, that is constrained by a synthetic brace, or staple), it has potential to be the best.
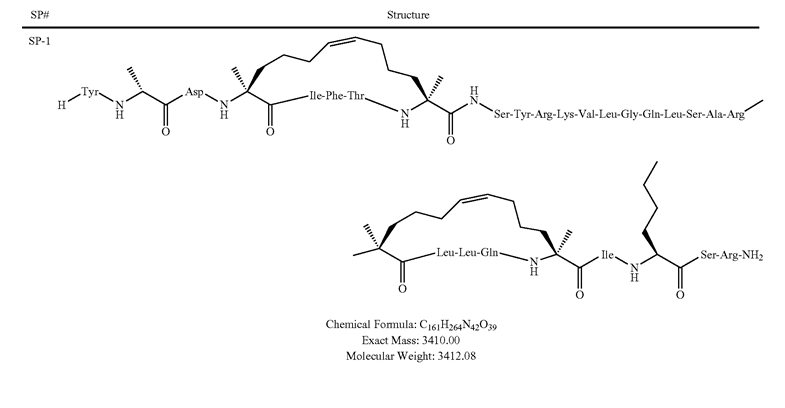 Stabilized helical peptides hold much promise as therapeutics in many human diseases. However, they have yet to be used clinically. This may be beginning to change. Two stapled peptides from Aileron Therapeutics, Inc., have been the subject of clinical trials. ALRN-5281 is a 29-amino acid analog of human growth hormone-releasing hormone (GHRH) that may be clinically applicable in patients with growth hormone deficiency syndromes.
Stabilized helical peptides hold much promise as therapeutics in many human diseases. However, they have yet to be used clinically. This may be beginning to change. Two stapled peptides from Aileron Therapeutics, Inc., have been the subject of clinical trials. ALRN-5281 is a 29-amino acid analog of human growth hormone-releasing hormone (GHRH) that may be clinically applicable in patients with growth hormone deficiency syndromes.
In 2013, the company announced the successful completion of the first in-human clinical trial of a stapled peptide drug (ALRN-5281) for the treatment of rare endocrine diseases such as adult growth hormone deficiency and HIV lipodystrophy. The main outcome of the trial showed ALRN-5281 had no incidence of adverse events in healthy subjects. In an abstract to the Endocrine Society’s 95th Annual Meeting and Expo in 2013, administration of ALRN-5281 demonstrated an increase of growth hormone in rats (administered intravenously) and dogs (administered twice weekly subcutaneously). These results supported the study of ALRN-5281 as a once-weekly injectable agent in the phase I study.
Due to its stapled nature, it will last incredibly long and is probably orally bioavailable. It looks like a great addition to GH treatment regimens.
Summary
Although many peptides and small molecules have stopped being widely available to physicians and patients due to recent FDA changes, peptides are still a quickly growing area of pharmaceutical development with the ability to help many conditions that are currently hard to treat.
The FDA’s enforcement of the bulk drug substance list will make these unavailable until they become FDA approved, but they are still worth knowing about and following if you are interested in any of these categories of treatment. And, as mentioned earlier, many physicians, at least for the time being, still seem to be able to get their hands on and prescribe a few of the peptides above, so in my opinion, it's worth at least asking your physician about.
There are breakthroughs happening daily as science continues to improve around drug molecule prediction and these products are put through the necessary and indispensable clinical trials. If you’d like to support the research and availability of some of these products, you can sign up for notifications here. SavePeptides.org is trying to bring many of the products I described above to be approved by the National Formulary (NF) and the United States Pharmacopeia (USP) monograph so they can be compounded by licensed pharmacies and distributed for individual patients with a prescription.
To find a doctor in your area who specialized in peptides, check out the International Peptide Society. If you want to learn more about peptides, here are some of my previous podcasts and articles on the subject:
- Demystifying Growth Hormone-Releasing Peptides – Everything You Need To Know About GH, Ipamorelin, Tesamorelin & More!
- Peptides Unveiled: The Best Peptide Stacks For Anti-Aging, Growth Hormone, Deep Sleep, Hair Loss, Enhanced Cognition & Much More!
- The Dark Side Of Peptides: Why You Need To Proceed With Caution When Using These Powerful But Potentially Carcinogenic Molecules.
- The Peptides Podcast: Everything You Need To Know About Anti-Aging, Muscle Gain, Fat Loss & Recovery Peptides.
- The Little-Known Russian Wonder Compound & The Fringe Future Of Anti-Aging Medicine.
- How To Use BPC-157: A Complete Dummies Guide To Healing The Body Like Wolverine.
- How To Use Growth Hormone Stacks For A Better Body: Everything You Need To Know About IGF-LR3, GHRP, and GHRH Peptide Stacks.
What about you? Have you used peptides in the past that have been affected by these recent changes? Do you have other questions? Leave your comments, thoughts, and questions below!













The FDA’s big scrutiny seems to come around whenever the pharmaceutical industry anticipates the release of a new drug that they want to ensure corners the market. However, it seems like there are obvious limitations to their ability to regulate, and in my opinion a good thing when it comes to prescription compounding.
For example, bremelanotide was approved by the FDA in 2019 as Vyleesi for female sexual arousal disorder. The same molecule, PT-141, has been available through compounding for years prior. If I remember correctly, Vyleesi, was around $1200 for a month supply around a year ago. On the other hand, PT-141 is much cheaper – better for everyone except the pharmaceutical company.
I think part of the issue they’re having with regulation is the low cost of production for many of these peptide molecules. Is this accurate?
Where are a couple of online (credible) places to buy peptides?
intelligentnutritioninc.com has BPC-157 compounded and manufactured in USA. I love it.
https://skyepeptides.com/ and https://aminos-research.com/ Both do a nice job of testing products.
Check out my Ultimate Peptides Resource —
https://shopbengreenfieldlife.mykajabi.com/peptides
Is it still legal to purchase these substances thru research chemical websites as long as they are stated “Not For Human Consumption”?
Also, how legit and safe are the substances from these sites?
Thanks and a pox on the FDA!
Internet websites are risky as “research only” products do not require preparation in a cGMP manner, nor are they regulated through FDA inspections … they could be made in a random basement somewhere in an unclean environment and not even be sterile … there’s no regulation for “research only” products other than the requirement that the label indicates “research use only, not for human consumption”. Compounding pharmacies are regulated through State and Federal agencies, must compound in aseptic environments and obtain their chemicals via an FDA inspected/approved vendor, ensuring that the chemical has at least some reason of quality. Also, compounding pharmacies will always dispense sterile peptides for injection … it’s unknown and not even stated if the product is sterile on the labels of internet peptides.
https://asm.org/
https://www.genengnews.com/
https://microbiologics.com/
https://regulatoryandmore.com/
https://asm.org/ASM/media/Policy-and-Advocacy/LRN/FDA-IVGuidance6-2011.pdf
How do we defund the FDA? They soo obviously are biased and lobbied by special interests, they have no actual interest in the safety of a product. These things can be proven, they barely attempt to hide it. So what’s the process of closing it down?
Not gonna happen, pharma corporations need the FDA to protect market share and revenues. It’s a way to keep the ball in their own court . . . . their ball.
Ben, I am in a dietetic internship now at Mississippi State University and do not have a lot of free time on my hands. Is it possible for your to share some of the key blogs you follow on feedly surrounding the hot topics like cutting edge nutritional research, research on supplements, anti-aging, athletic performance, sleep, lifestyle, spirituality, etc.? I want to drastically cut down my time per week looking for quality findings. Thanks so much for any guidance!! Have a great day!
Hi Ben, thanks for the article and the great information on the new peptides. Do you have any information as to where the push came from to make the FDA change the regulations to shut down our access. Obviously it is financially related – but is it that BIG Pharma wants to increase market share or just shut down our access and push people to use their drugs?
Is there a congressman behind a bill on this issue? I cannot find anything on the causal factors except what i think is the reason.
Thanks!
Thank you so much for putting out this information Ben! I suffer with IBD and I have used BPC-157 for treatment for the past two years, but I did not know about Larazotide or MCC950. I am staying as current as possible with clinical trial developments for new drugs and supplements as ways to support my gut before a flare would / could occur. I am young and have lost many years of my life due to this awful disease and your research has helped so very much minimize the duration of flare-ups I have. Thank you again for your exceptional work. I appreciate you and your team.
Great and promising news! Thanks for the update and overview of these relatively obscure peptides.